Abstract
Background. Binet A CLL, which includes most newly diagnosed cases, has considerable heterogeneous disease courses. Some patients can live for decades without requiring therapy, while others require therapy within months from diagnosis. Given the improved tolerability and efficacy of newer agents and combinations, it is important to identify patients with CLL who, despite initially presenting with early stage CLL, are at increased risk for accelerated progression to symptomatic disease. Such patients are candidates for early therapeutic intervention trials testing whether biomarker-triggered treatment rather than symptom-triggered treatment may improve overall outcome in CLL, as already documented in the myeloma setting. On these bases, a specific and robust time to progression predictive model is needed.
Aim. Develop of a model for prognostication of time-to-first treatment (TTFT) in Binet A CLL.
Methods. We performed an analysis using individual patient data from clinically and biologically annotated Binet A CLL cohorts initially managed with watch and wait, including institutional series, clinical trial series, and population series, accounting for a total of 2816 patients with a median follow-up ranging from 5 to 12 years. The adjusted association between exposure variables and TTFT was estimated by Cox regression. Backward elimination using likelihood ratio statistics with selection criterion p <.05 was used to derive the final model. We assigned a weighted risk score to each factor of the final model based on the regression parameters from the Cox regression analysis. The prognostic score was then defined as the sum of single-risk parameters. We identified different risk groups based on recursive partitioning. Discrimination capacity of the model was assessed through c-index.
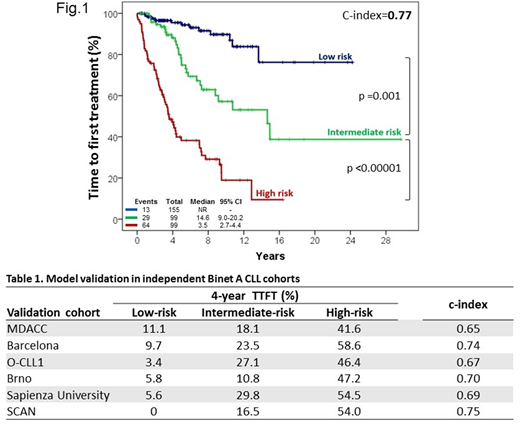
Results. The training cohort included a single-institution series of 353 consecutive Binet A CLL from the University of Eastern Piedmont. Nineteen baseline markers were considered as covariates, including clinical (age, sex, palpable lymph nodes, palpable spleen), laboratory (haemoglobin, platelet and lymphocyte count, beta-2-microglobulin), cytogenetic [del(17p), del(11q), trisomy 12, del(13q)], and molecular (IGHV, ATM, MYD88, NOTCH1, SF3B1, TP53 mutations) variables. By multivariable analysis, only five variables were independently associated with TTFT and were used to build the prognostic score, namely: lymphocyte count >15 G/L (HR=2.5), palpable lymph nodes (HR=2.4), palpable spleen (HR=2.4), unmutated IGHV gene (HR=2.8) and trisomy 12 (HR=2.4). Using weighted grading, a score of 1 was assigned to each variable. A prognostic index was derived, separating three different groups: low- (score 0), intermediate- (score 1), and high-risk (score 2-5) with significantly different probability of need of therapy (4.5%, 11.9%, 52.8% at 4 years for the low- to high-risk group, respectively, c-index: 0.77; Fig. 1). The model was consistently confirmed in 6 validation series (Table 1), including: i) 1225 Binet A patients from the MDACC institutional cohort; ii) 332 patients from the Barcelona institutional cohort; iii) 283 patients from the O-CLL1 trial (NCT00917540); iv) 241 patients from the Brno institutional cohort; v) 205 patients from the Sapienza University institutional cohort; and vi) 177 patients from the SCAN population-based cohort.
Conclusion. The resulting model combines routine clinical and molecular variables in an easily applicable prognostic score for estimating risk for progression to active CLL requiring treatment among early stage CLL. This model was validated by international data sets of Binet A patients and can help design of clinical trials testing whether therapy initiation at an earlier stage than at manifestation of organ complications is beneficial for high-risk patients.
Doubek:Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Affimed: Research Funding; Gilead: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Novartis: Consultancy. Mauro:janssen: Other: board member; abbvie: Other: board member. Zucca:Celltrion: Consultancy; AstraZeneca: Consultancy. Foà:CELTRION: Other: ADVISORY BOARD; INCYTE: Other: ADVISORY BOARD; NOVARTIS: Speakers Bureau; GILEAD: Speakers Bureau; AMGEN: Other: ADVISORY BOARD; JANSSEN: Other: ADVISORY BOARD, Speakers Bureau; ABBVIE: Other: ADVISORY BOARD, Speakers Bureau; CELGENE: Other: ADVISORY BOARD, Speakers Bureau; ROCHE: Other: ADVISORY BOARD, Speakers Bureau. Gaidano:Roche: Consultancy, Honoraria; Morphosys: Honoraria; Gilead: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Wierda:AbbVie, Inc: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal